Overview
At UTRGV, my goal is to establish a cardiovascular biomechanics research program that advances patient care through innovation. My work focuses on developing simulation-based technologies to optimize medical device design, improve treatment planning, and enable patient-specific risk assessment. Through close collaboration with clinicians and multidisciplinary teams, I aim to create transformative solutions that enhance cardiovascular healthcare outcomes.
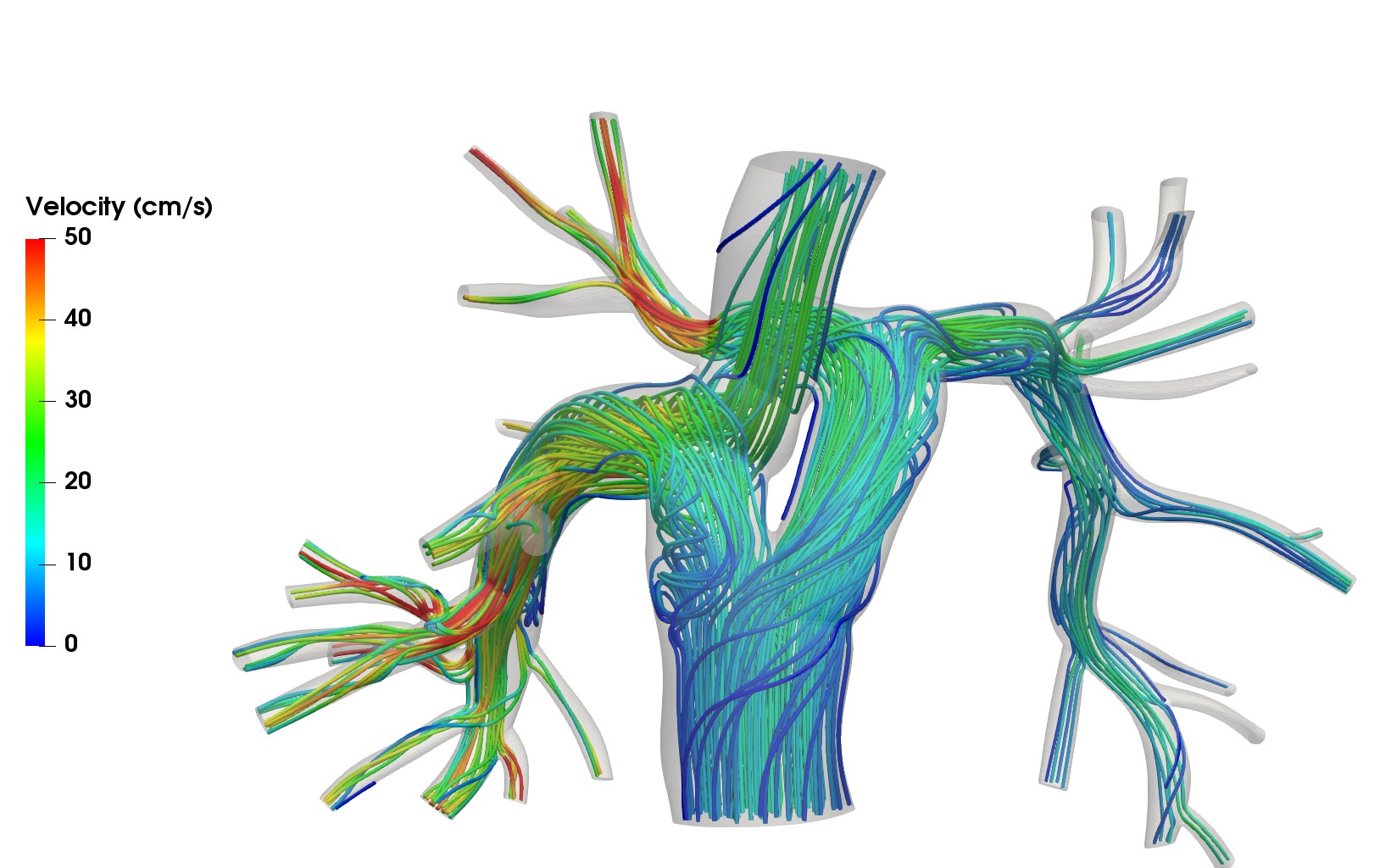
The Y-Shaped Fontan Graft
Single-ventricle heart defects result in only one functional pumping chamber, necessitating surgical rerouting of blood flow. The Fontan procedure directs venous return passively to the pulmonary arteries. We developed a novel Y-shaped graft, optimized through computational modeling to enhance circulation efficiency. A pilot study has demonstrated the feasibility of this approach through the first human implantations of the Y-graft. Collaborators: Drs. Jeffrey Feinstein and Alison Marsden, Stanford University.
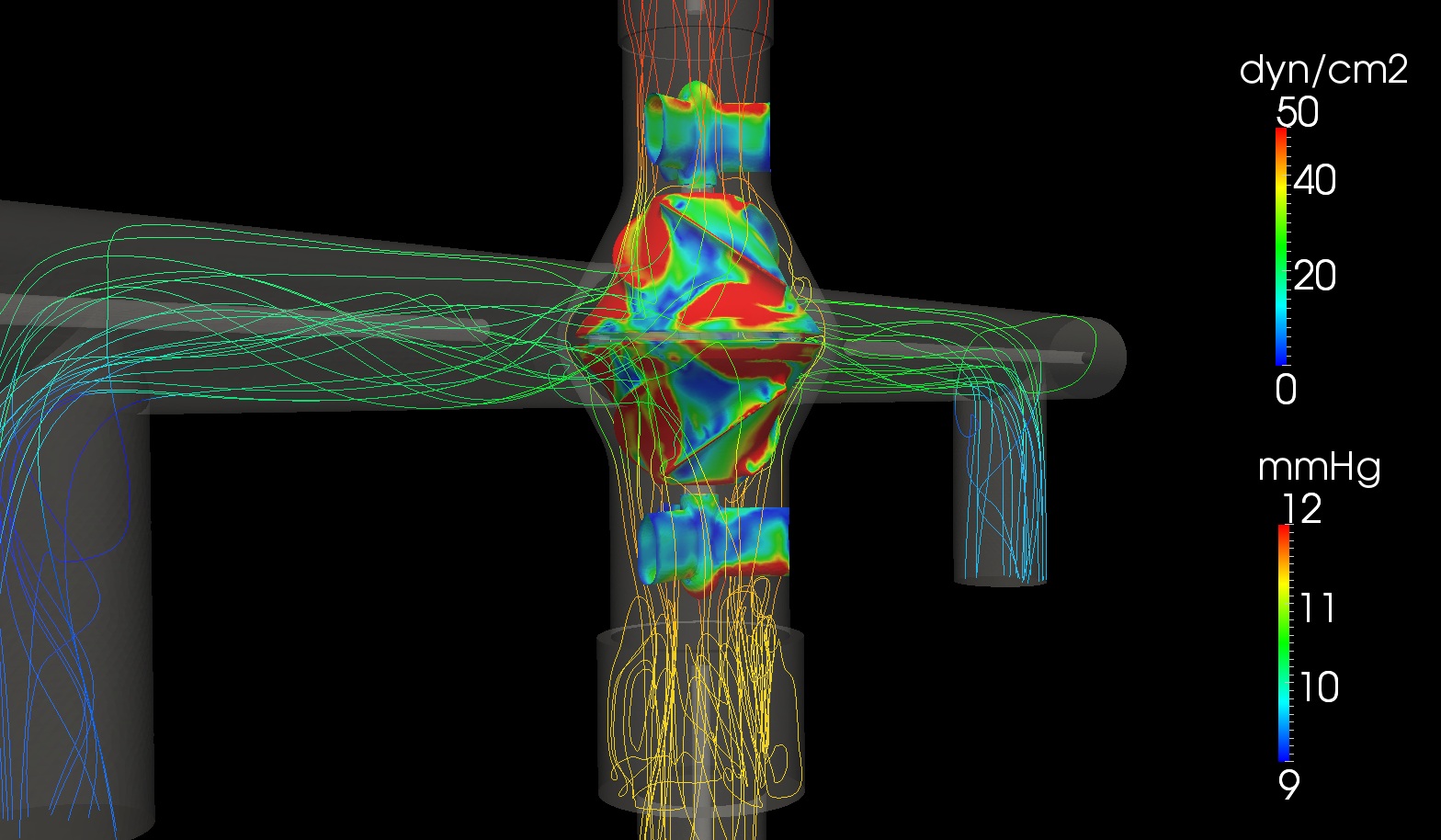
Viscous Impeller Pump
Despite being a life-saving procedure, the Fontan circulation often fails over time due to the absence of a sub-pulmonary ventricle. To address this limitation, we are developing a viscous impeller pump (VIP) designed specifically for the unique physiology of Fontan patients, aiming to restore near bi-ventricular function and improve long-term outcomes. In collaboration with Dr. Mark Rodefeld at Indiana University, we are advancing pump design to ensure passive safety in the event of pump failure.
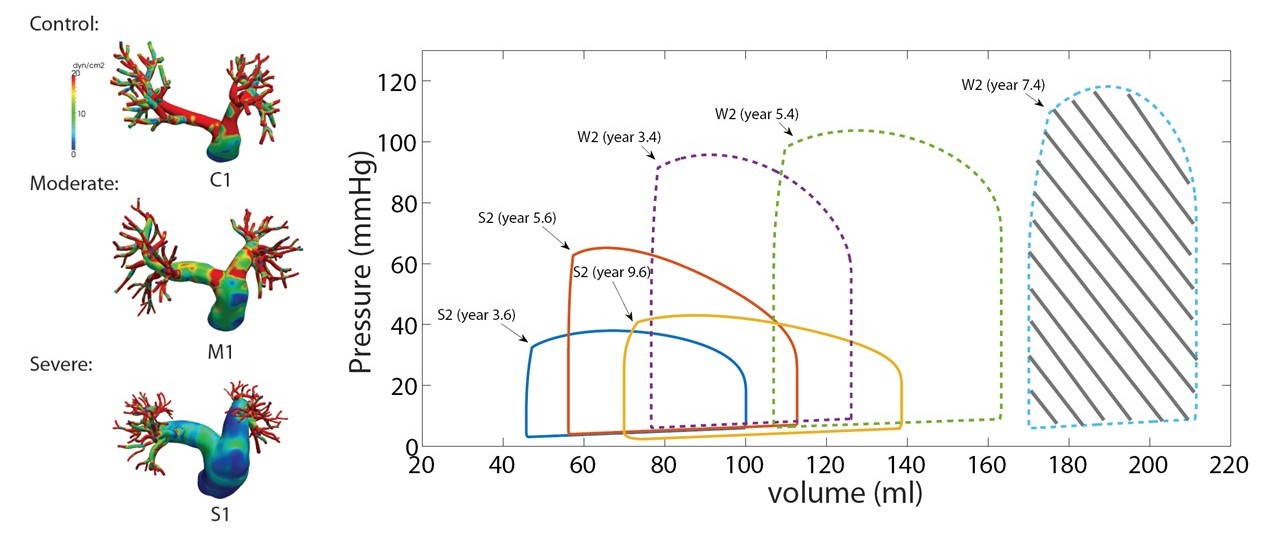
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a progressive vascular disease that elevates pulmonary pressure and imposes excessive load on the right ventricle, leading to heart failure in children. Using patient-specific simulations and mathematical modeling, we investigate the hemodynamic forces and ventricular workload driving disease progression. Our studies revealed evolving wall shear stress patterns with worsening PAH and showed that right ventricular stroke work correlates strongly with clinical outcomes. These findings identify biomechanical biomarkers for disease severity and inform improved diagnostic and treatment strategies. Collaborators: Drs. Jeffrey Feinstein, Alison Marsden, and Marlene Rabinovitch, Stanford University.
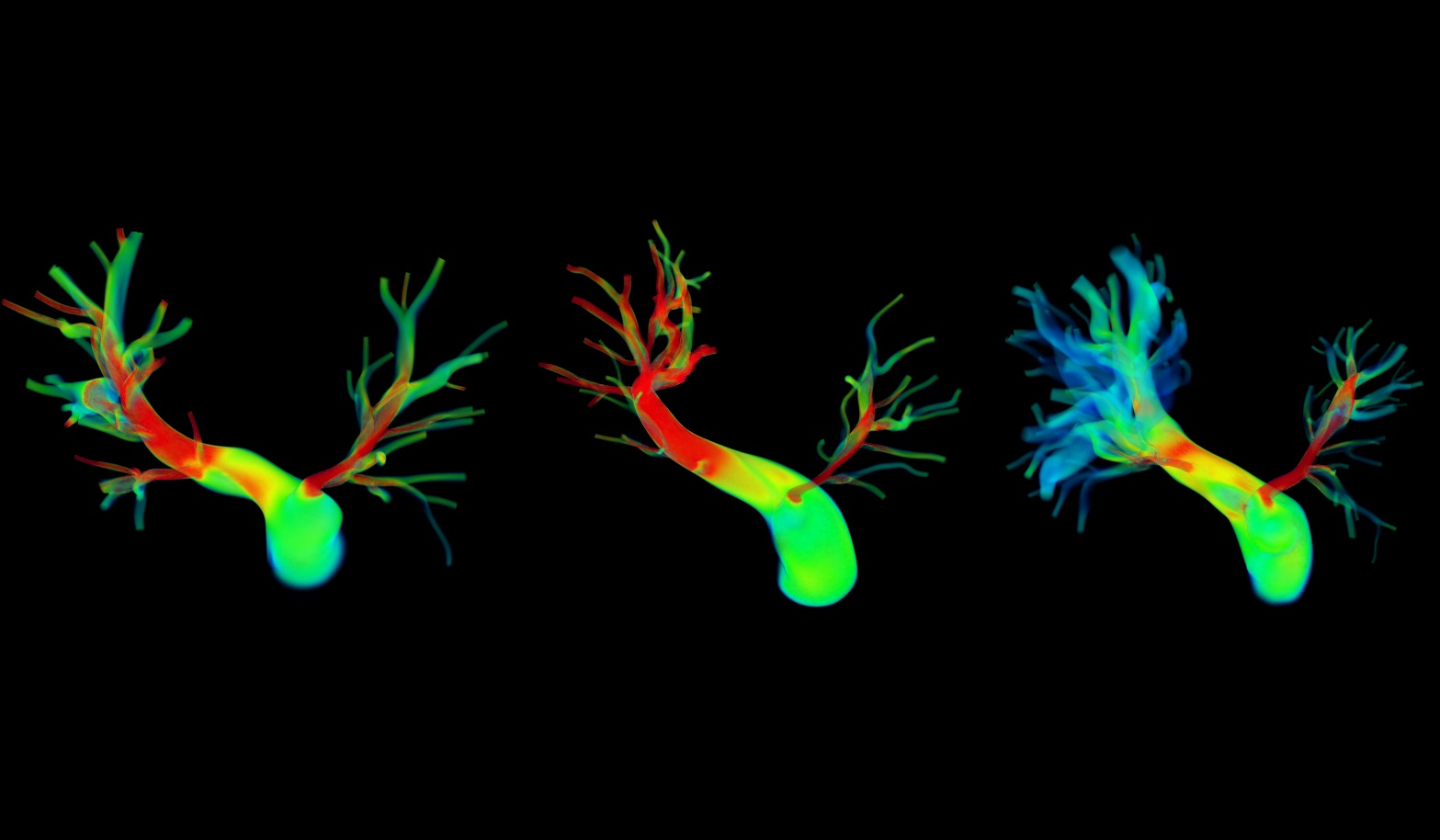
Peripheral Pulmonary Artery Stenosis
Peripheral pulmonary artery stenosis (PPS) is characterized by the narrowing of the pulmonary arteries that increases vascular resistance, leading to pulmonary flow disparity and right ventricular hypertension. Our research applies patient-specific computational modeling to simulate pre- and post-operative hemodynamics and evaluate outcomes following surgical or catheter-based interventions. We accurately predict post-treatment flow distribution and pressure recovery. These simulations support virtual treatment planning, enabling clinicians to assess alternative intervention strategies and optimize outcomes for complex PPS. Collaborators: Drs. Jeffrey Feinstein, Frank Hanley and Alison Marsden, Stanford University, and Dr. Irene Vignon-Clementel, INRIA, France.