PARAMXYOVIRIDAE
General Characteristics
Morphology
- Non-segmented, enveloped, (-) ssRNA Group V
- Virions are 150-300 nm in diameter.
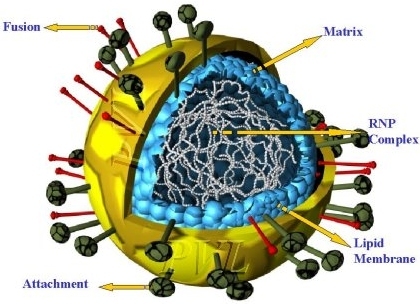
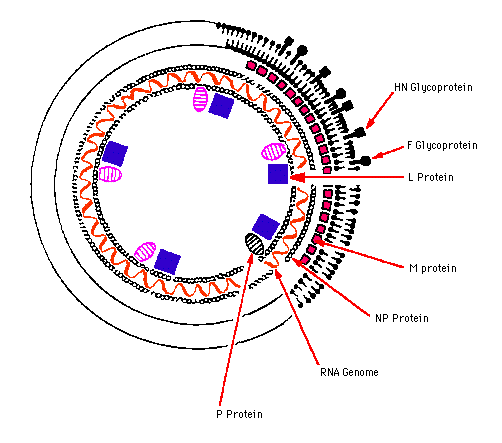
- Virions have a complex construction and consist of an envelope, a nucleocapsid, and a matrix protein.
- Irregular (pleomorphism) but usually spherical in shape (filamentous forms also occur).
- Envelope with large peplomers 8-12 nm in length.
- Helical flexible nucleocapsid (13-18 nm diameter).
- Matrix proteins inside the envelope stabilize virus structure.
- The nucleocapsid core is composed of the genomic RNA, nucleocapsid proteins, phosphoproteins and polymerase proteins.
- Two glycosylated envelope proteins: a fusion protein (F) and an attachment protein (G, H or HN).
- The surface glycoproteins of some viruses have neuraminidase activity (e.g. Paramyxovirus and Morbillivirus),and fusion activity (all genera).
- Have narrow host ranges; they have only been conclusively identified in vertebrates, mostly in mammals and birds.
- Transmission is horizontal mainly by aerosols and droplets.
- Sensitive to heat, lipid solvents, nonionic detergents, formaldehyde, and oxidizing agents.
- The virus is probably distributed worldwide.
Source: ICTV
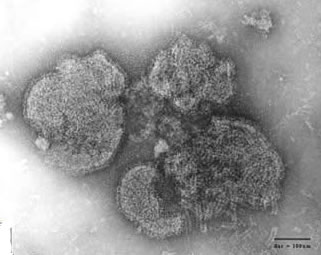
Source: S. McNulty, Queens University, Belfast

Source: L. Stannard, University of Cape Town
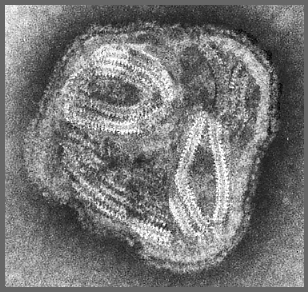
Source: L. Stannard, University of Cape Town

The helical ribonucleo-protein capsid looks like a "herring-bone"
Taxonomy
Order: Mononegavirales (the non-segmented, negative-stranded RNA viruses)
Family: Paramyxoviridae
Subfamily: Paramyxovirinae
Genera: Paramyxovirus
Rubulavirus
Morbillivirus
Henipavirus
Subfamily: Pneumovirinae
Genus: Pneumovirus
The term myxo (Gr. myxa: mucous, slime) identifies the specific affinities or ortho and paramyxoviruses for mucopolysaccharides and glycoproteins, particularly sialic acid-containing receptors on the cell surfaces.
In the case of the influenza virus (Orthomyxovirus - segmented genome) this mucus is at the mucous membrane of the respiratory tract.
Genome
Virions contain 10-12 proteins.
Consists of a single molecule of linear, (negative sense), ss RNA, 16-20 kb in size.
Three nucleocapsid-associated proteins common in all genera
N or NP, an RNA-binding protein
P, a phosphoprotein
L, a large putative polymerase protein
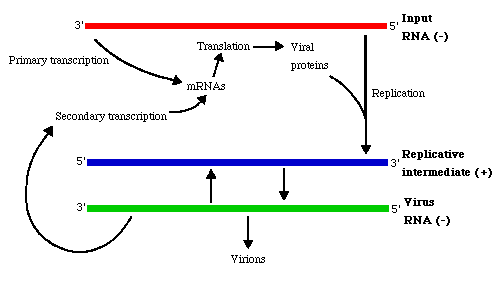
The genome is transcribed from the 3'-end by virion-associated enzymes into 6 to 10 separate, subgenomic, viral-complementary mRNAs.
The 5'-end of the negative-sense strand does not have a covalently attached terminal protein; genome does not have cap or poly (A) tract.
The mRNA are capped and have a 3'-poly(A) tract.
The genome is not infectious (by itself).
Antigenic determinants may be found on peplomers of envelope and correspond to each of the major virion proteins [attachment protein (HN, or H, or G), and fusion protein (F)].
Attachment and Formation of Syncytia
The envelope possesses haemagglutinin (HA) spikes that can hemagglutinate or bind to specific erythrocytes. The envelope also has neurominidase (NA), an enzyme that breaks down the sialic acid component of the membrane. Sialic acid is a derivative of N-acetyl-neuraminic acid. Sendai virus has spikes with both NA and HA. Pneumovirus has neither activity. Meales virus (Morbillivirus) has HA spikes but no NA activity. Cell membrane of an infected cell is modified by insertion of the spikes. F glycoproteins spikes allows virus to infect neighboring cells by a novel mechanism: "Fusion from within" or endocytosis. Trypsin-like proteases on the cell surface can activate the F protein to cause fusion with nearby cell surfaces. The HN spikes immediately bind an uninfected neighboring cell, and in the presence of F spikes, the two cells permanently fuse. The chain reaction of multiple cell fusions then produce a syncytium (Gr. Syn, together; kytos, cell) or multinucleate giant cell, with cytoplasmic inclusion bodies. The fusion allows direct passage of viruses from an infected cell to uninfected cell by communicating membranes. Through this means, the virus evades antibodies.
Source: Microbiology-bytes
Replication
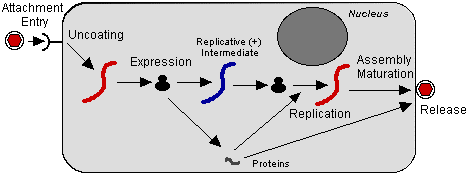
- Takes place in the cytoplasm and assembly occurs via budding on plasma membranes.
- RNA-dependent RNAP is involved in the conversion of a negative to a positive strand, and an RNA endonuclease, cuts a primer from capped mRNA precursors.
- Negative strand RNA viruses encode and package their own RNA transcriptase, but mRNAs are only synthesized once the virus has been uncoated in the infected cell.
- Monocistronic mRNAs transcribed from genome by RNAP that is part of virion.
- Viral replication occurs after synthesis of the mRNAs and requires the continuous synthesis of viral proteins.
- The newly synthesized antigenome (+) strand serves as the template for further copies of the (negative) strand genomic RNA.
- After primary transcription, plus-strand templates for the formation of progeny minus-strand molecules are formed.
- Details of assembly are still uncertain.
Source: Microbiology-bytes
Human Pathogens
Genus: Paramyxovirus
Human parainfluenza viruses 1 and 3
Genus: Henipavirus
Hendra virus
Genus: Rubulavirus
Mumps virus, human parainfluenza viruses 2, 4a, 4b
Genus: Morbillivirus
Measles virus
Genus: Pneumovirus
Human respiratory syncytial virus
Human Parainfluenza Viruses (HPIVs)
A group of four distinct serotypes of ssRNA viruses belonging to this viral family.
Serotypes:
HPIV-1 (most common cause of croup; also other upper and lower respiratory tract illnesses typical)
HPIV-2 (causes croup and other upper and lower respiratory tract illnesses)
HPIV-3 (associated with bronchiolitis and pneumonia)
HPIV-4 (includes subtypes 4a and 4b)
They are the second most common cause of lower respiratory tract infection in younger children.
Reinfection with parainfluenza viruses is rare, so secretory immunoglobulins must create effective immunity.
Efforts to make a vaccine for parainfluenza viruses have not been successful.
Croup (acute obstruction of the larynx) produces a harsh cough that sounds like a seal's bark.
Animal Pathogens
Genus: Paramyxovirus
Bovine parainfluenza virus Type 3
Mouse parainfluenza virus Type 1 (Sendai virus)
Simian parainfluenza virus Type 10
Genus: Rubulavirus
Newcastle-disease virus (avian paramyxovirus)
Avian paramyxovirus 2 (Yucaipa virus)
Avian paramyxoviruses 3, 4, 5, 6, 7, 8, 9
Porcine rubulavirus (La Piedad Michoacan-Mexico virus)
Simian parainfluenza viruses 5 and 41
Genus: Morbillivirus
Canine distemper virus
Rinderpest virus
Peste-des-petits-ruminants virus (goats and sheep)
Equine morbillivirus (Australia)
Dolphin distemper virus
Porpois distemper virus
Phocine (seal) distemper virus
Genus: Pneumovirus
Bovine respiratory syncytial virus
Pneumonia virus of mice
Turkey rhinotracheitis virus
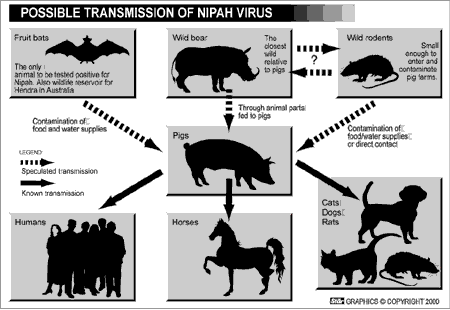
Nipah and Hendra Viruses: New Emerging Animal Viruses
Hendra virus (now an Henipavirus but formerly called equine morbillivirus) was first isolated in 1994 from specimens obtained during an outbreak of respiratory and neurologic disease in horses and humans in Hendra, a suburb of Brisbane, Australia.
Nipah virus, also a member of the family Paramyxoviridae, is related but not identical to Hendra virus.
Nipah virus was initially isolated in 1999 upon examining samples from an outbreak of encephalitis and respiratory illness among adult men in Malaysia and Singapore. Its name originated from Sungai Nipah, a village in the Malaysian Penninsula where pig farmers became ill with encephalitis.
Nipah and Hendra viruses have emerged as new pathogens in Malaysia and Australia respectively in recent years.