Subviral Particles: Satellite viruses
Satellite Virus
Satellites are small RNA molecules (500 - 2000 nt) which are dependent on the presence of a helper virus for replication but unlike defective viruses (viroids), show no nucleotide sequence homology to the helper virus genome.
Larger satellite RNA's may encode a protein.
Characteristics
- Contain unconventional nucleic acid genomes, but their replication
is dependent on the presence of a
helper virus that provides the replicase (helper-virus encoded replicase).
- Satellite virus is not required for the helper virus replication.
- Do not exhibit substantial sequence homology with their helper
viruses.
- All known satellite viruses encode their own capsid proteins.
- The interdependence is reminiscent of that between hepatitis
delta virus (a viroid or defective virus) and HBV.
- Adeno-associated virus and Adenovirus
(Dependoviridae).
- The helper virus for satellite tobacco necrosis virus (STNV)
is tobacco necrosis virus (TNV), a 30 nm icosahedral plant virus.
- Some satellite RNAs encode proteins; these are called satellite
viruses and, like virusoids, must co-infect with a helper virus
in order to replicate.
The differing replication strategies among the groups of subviral particles reflect different evolutionary origins.
Subviral pathogens represent new frontiers for research.
Virusoids
- Virusoids belong to a larger group of infectious agents called
satellite RNAs, which are found in bacteria,
plants, fungi, invertebrates, and vertebrates.
- A virusoid is an infectious agent that infects plants in conjunction
with an assistant virus; the assistant virus
harbors the virusoid and is required for successful infection.
- Virusoids consist of a single molecule of single-stranded circular
RNA that is several hundred nucleotides long
(220-388) and codes for nothing but its own structure.
- In size and structure,
virusoids are similar to viroids (circular RNA molecules that
don't require an assistant virus).
- Like a viroid but virusoids are wrapped in the helper virus's
capsid.
Replication of Virusoids
- A virusoid genome does not code for any proteins, but instead
serves only to replicate itself.
- Virusoids can replicate in the cytoplasm
and possess a ribozyme (self-cleavage) activity.
- RNA replication is similar to that of viroids, but each requires
that the cell be infected with a specific "helper" virus.
- Five virusoids are known, and the helper viruses for these
are all members of the Sobemovirus
family.
- An example of a "helper" virus is the subterranean clover mottle
virus, which has an associated virusoid.
- Virus enzymes may aid replication of the virusoid RNA.
- The virusoid is incorporated into the virus particle and transmitted
as a "satellite," a separate nucleic acid not part of the viral
chromosome.
- Replication of the helper virus is independent of the virusoid.
Examples of Satellite RNAs
Cucumovirus
Cucumber mosaic
Peanut stunt
Carmovirus
Turnip crinkle D
Nepovirus
Tobacco ringspot
Grapevine fanleaf
Tomato black ring
Chicory yellow mottle
Sobemovirus
Velvet tobacco mottle
Lucerne transient streak
Luteovirus
Barley yellow dwarf
| Characteristic | Virus | Viroid | Virusoid | Delta Agent |
| Nucleic acid | ss, dsDNA ss, dsRNA | ssRNA | ssRNA | ssRNA |
| Circular RNA | no | yes | yes | yes |
| Size (in bases) | 4 -- 500,000 | 246-375 | 350 | 1,678 |
| Replication | autonomous | autonomous | helper | helper |
| Rolling circle replication | yes (in some) | yes | no | yes |
| Capsid | yes | no | no | in helper's capsid |
| Genes | yes | no | no | yes |
| Need for helper virus | no (only Parvoviridae) | no | yes | yes |
| Viewed by | electron microscope | nucleotide sequence | nucleotide sequence | host cell damage |
| Host | microbes, plants, animals | plants | plants | mammals |
Virinos
A hypothetical ultra-small infectious particle thought to be the
cause of scrapie and other degenerative diseases of the
central nervous system; consists of nucleic acid in a protective
coat of host cell proteins.
Some scientists still think encephalopathies are caused by 'dementia viruses' (virinos) and not by infectious proteins (prions).
Non-Viral Particles: Nanobes
Properties
- Nanobes are tiny filamental structures first found in some rocks
and sediments.
- Has a morphology similar to Actinomyces
and Fungi.
- Though still rudimentary, research evidence indicates the existence
of microorganisms below the size of the
smallest bacteria and more complex than viruses.
- The subviral pathogens have been named nanobes
because their size ranges from about 20 to about 100 nm (the smallest
bacteria are about 150 nm).
- They may reproduce via some unconventional means, like RNA instead
of DNA.
- The Martian meteorite ALH84001, discovered in 1984 in the Antarctic,
contained similar tubular structures which some astrobiologists
suggested could be evidence of life at an earlier time on Mars.
- Researchers have found evidence of their presence in patients
with large cysts in their kidneys (renal stones).
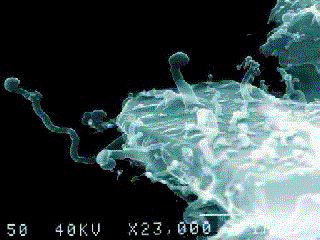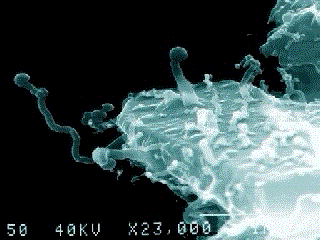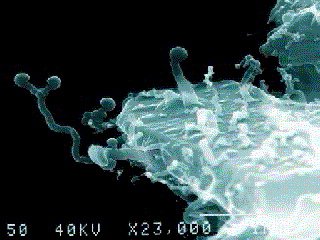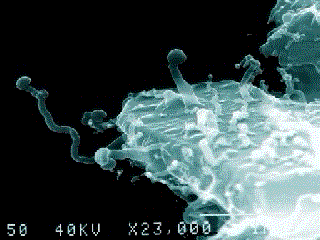
A nanobe (Photo by Dr. P. Uwins)
Virus-Like Infectious Agents: Prions
- Prions are described as proteinaceous infectious particles thought
to cause a number of diseases
(kuru, mad cow disease, scrapie).
- Prions were named in 1982 by Stanley B. Prusiner, winner of
the Nobel Prize in Physiology/Medicine.
- The award surprised the scientific community because this prize
is generally awarded to two or more individuals in each category,
and because the work on prions remains somewhat speculative and
incomplete.
- Occasionally the term prion is used by some investigators as
a synonym for the infectious agent and for
PrP protein.
- Adriano Aguzzi and Charles Weissman (1997) provide a useful
definition of a prion: a prion is the agent of
transmissible spongiform encephalopathies (TSE) with unconventional properties.
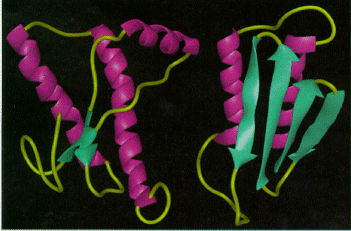
Normal and diseased prions.
The abnormal form (prion) consists
primarily of pleated sheets of misshapen protein that tend to
clump together.
Characteristics
- Tests indicate that prions can survive the heat, radiation,
and chemical treatment that normally inactivate viruses.
- Appear to be composed only of protein, because they are susceptible
to some protein-digesting enzymes but
not nucleases.
- These factors indicate that prions are not viruses and raise the questions of how prions replicate, because one of the central dogmas in biology is the inheritance operates through nucleic acids.
The Role of Prions in Disease
- Apparently they are deviant versions of a harmless protein found
on the membrane surfaces of most mammalian cells, particularly
brain cells.
- It is called prion protein (PrP
gene).
- Researchers in 1997 have reported that the role of PrP
is to bind copper ions and thereby help the cell resist the toxic
effects of highly reactive oxygen radicals.
- Their evidence shows that after exposure to copper, the brain
cells of mice lacking PrP
die more readily that do normal brain cells.
- Apparently the enzyme that protects cells from oxygen radicals
(superoxide dismutase) requires copper for its activity.
- Without PrP, no copper is bound, and the enzyme is inactive, thereby leading to toxicity and cell death
The Two Types of PrP
In the normal state, the PrP protects its cell.
Then a transformation occurs, and the PrP folds into a different form: a prion.
By folding into an aberrant shape, the normal prion turns into a rogue agent. It then induces other normal prions to become rogue prions.
In the normal form, PrP is soluble in some detergents and can be digested by protease K; but in the abnormal form, the prion is insoluble in those detergents and resistant to proteases.
The normal protein PrPC (c for cellular) is a 33-35 kdaltons molecule.
The abnormal protein PrPSc (Sc for scrapie) is 27-30 kdaltons.
Aggregates into the macro- molecular amyloid fibrils associated with the disease.
The abnormal form (prion) consists primarily of pleated sheets of misshapen protein that tend to clump together.
In doing so, they may block the molecular traffic of cells by the sheer force of large size and eventually kill the cells.
Cellular Damage Caused by Prions
Brain tissues develop a sponge-like appearance with empty areas of dead tissue.
These are the transmissible spongiform encephalopathies (TSEs).
TSEs are an array of slowly progressing, inevitable fatal, diseases that gradually destroy the central nervous system.
TSE Diseases of Animals
- Scrapie
In sheep and goats.
Recognized for 200 years.
Affects 0.5 to 1% of the sheep production in the UK per year.
- Transmissible mink encephalopathy (TME)
In minks
- Chronic wasting disease (CWD)
In deer and elk.
- Bovine spongiform encephalopathy (BSE)
In 'mad cow disease'.
- Feline spongiform encephalopathy (FSE)
In domestic and great cats.
- Exotic ungulate encephalopathy (EUE)
In nyala and greater kudu.
The Scrapie Disease Prion
- Scrapie infectivity was considerably more resistant than most
viruses to UV and ionizing radiation.
- The scrapie agent is 200-fold more resistant to UV radiation
than polyomavirus and 40-fold more resistant
than a mouse retrovirus.
- The scrapie agent is also more resistant to chemicals, such
as 3.7% formaldehyde, and autoclaving routinely
used to inactivate viruses.
- It is possible to reduce by 90 to 95% after several hours of such treatments, but some residual infectivity remains.
TSE Diseases of Humans
- Kuru
May have originally been established in the small population of Fore people in New Guinea by eating the brain of an individual with sporadic CJD.
- Creutzfeldt-Jacob disease (CJD)
Affects 1-2 million people worldwide, usually late in life (50-70).
There are two types: inherited (familial) and non-inherited (sporadic CJD).
- Fatal familial insomnia (FFI)
- Gerstmann-Straussler syndrome (GSS)
Unclear Questions Still Remain
- By this date, researchers have not yet shown how the transformation
from normal to abnormal PrP takes place.
- The question of normal PrP's function waits to be confirmed.
- How prions transfer from host to host and how they bring about
disease symptoms has not been established.
- Absolutely pure, synthetic prions (free of any possible contaminating
substance) have not yet been shown
to be capable of causing disease.
- Some believe that prions are shrouds of protein enclosing ultra-small
viruses (virinos).
- Further research is needed to clarify these issues.